By using MoodChangeMedicine.com, you agree to accept this website’s terms of use, which can be viewed here.
June 8, 2024
By Joie Meissner ND, BCB-L
SNAP SHOT ON St. JOHN’S WORT SAFETY
- When taken orally for up to 12 weeks in appropriate doses, St. John’s wort is likely safe, according to experts. 1, 2 But the herb may not be safe for people with certain medical conditions and those who regularly take medication.
- Taking St. John’s wort during pregnancy may cause birth defects
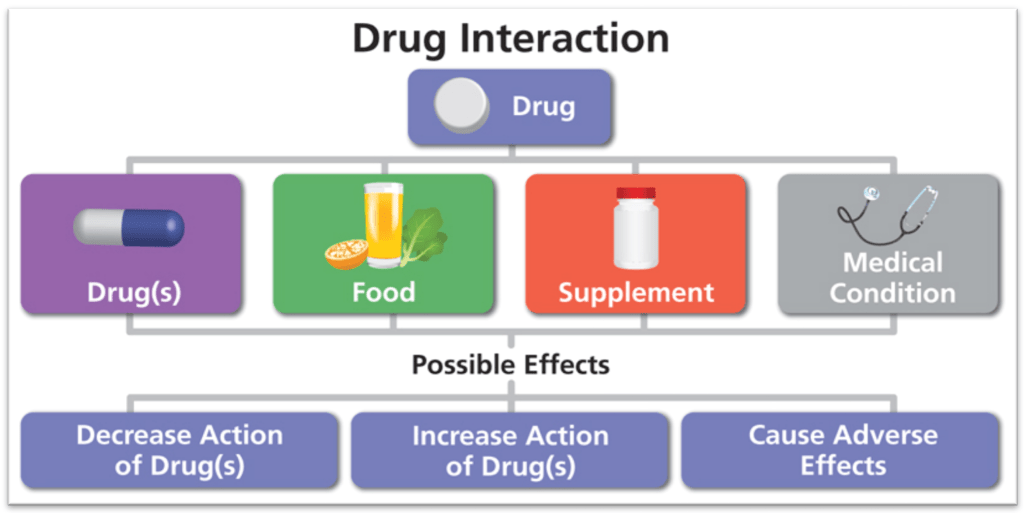
- Because St. John’s wort interacts with many drugs, it might not be safe for those who take pharmaceutical medications. For example, taking St. John’s wort in combination with birth control pills may lead to unplanned pregnancy. Taking St. John’s wort in combination with drugs that prevent organ transplant rejection could lead to loss of the transplanted organ. Taking it with blood thinners prescribed to prevent stroke may decreased the medication’s ability to reduce the risk of stroke. There are numerous other such examples.
American College of Physicians warn against that taking St. John’s wort in combination with certain drugs—monoamine oxidase inhibitors like isocarboxazid (Marplan), phenelzine (Nardil), selegiline (Emsam) and tranylcypromine (Parnate) or selective serotonin reuptake inhibitors (SSRIs) like citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil) and sertraline (Zoloft). 3 - Taking St. John’s wort before surgery is likely unsafe.
- It is likely unsafe to take St. John’s wort in high doses as it might be toxic.
- St. John’s wort may cause increased sensitivity to sunlight, especially when taken in larger doses.4 Symptoms include burning rash on sun-exposed skin.
- “St. John’s wort is generally well tolerated,” according to an expert, peer-review report. 5
- Side effects of St. John’s wort resemble that of antidepressant drugs, but studies show the herb’s side-effects are more tolerable than the side effects of the pharmaceuticals. 6
- St. John’s wort causes sexual dysfunction less frequently than SSRIs antidepressants like Zoloft. 7
- Similar to pharmaceutical antidepressants like Prozac, St. John’s wort may have withdrawal symptoms. 8, 9
- Rare side effects include changes in blood pressure.
- Other rare side effects could include hypomania, which could result in increased energy and activity levels, insomnia and lapses in judgement.
- Rare case reports of suicidal thoughts after taking St. John’s wort have appeared in the medical literature.
Always consult a physician before taking St. John’s wort.
St. John’s wort is likely safe “when used orally and appropriately. St. John’s wort extracts in doses up to 900 mg daily seem to be safe when used for up to 12 weeks. Some evidence also shows that St. John’s wort can be safely used for over one year,” according to an expert panel at NatMed Pro. 10
These experts state that due to the risk of severe phototoxic skin reactions, St. John’s wort is possibly unsafe “when used orally in large doses.” They further state that “taking 2-4 grams of St. John’s wort extract (containing hypericin 5-10 mg) daily appears to increase the risk of photosensitivity.” 11 In photosensitivity, the skin becomes very sensitive to sunlight or other forms of ultraviolet light and may burn easily. In phototoxic skin reactions, skin rashes may develop on sun-exposed areas.
“St. John’s wort is generally well tolerated”, according to experts at NatMed Pro. Serious side effects include rare case reports of suicidal thinking and loss of touch with reality. 12
Taking St. John’s wort during pregnancy is unsafe and may lead to fetal abnormalities. 13,14
The information provided on this site is for educational purposes only; it is not medical advice and should not be substituted for professional medical advice.
Special Safety Concerns
St. John’s wort can interact with a variety of medications in potentially dangerous ways. It can make many medications less effective. For example, it could make hormonal birth-control pills less effective. It can make other medications more potent so that the medication’s side-effects or toxicity are increased. St. John’s wort may decrease the efficacy of chemotherapy, radiation therapy or anesthetics used in surgery. 15
If St. John’s wort is combined with certain antibiotics, pain and headache medications or antidepressant drugs like Prozac, and Zoloft, it could dangerously elevate serotonin levels. Combinations with other serotonin-boosting supplements might also dangerously elevate serotonin levels.
Serious, potentially life-threatening, side-effects might occur as a result of taking St. John’s wort with other medications that also boost serotonin including monoamine oxidase inhibitors like isocarboxazid (Marplan), phenelzine (Nardil), selegiline (Emsam) and tranylcypromine (Parnate) or selective serotonin reuptake inhibitors (SSRIs) like citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), paroxetine (Paxil) and sertraline (Zoloft). These combinations are contraindicated. 16
St. John’s wort’s use during pregnancy or while breastfeeding is risky. St. John’s wort given to lab animals resulted in birth defects. There have been reports of infants who are breastfeeding becoming fussy, drowsy, or having colic when their mothers have taken St. John’s wort.
Taking St. John’s wort before surgery is likely unsafe due to potentially dangerous interactions with anesthesia causing serious cardiovascular problems.
St. John’s wort might not be safe in patients with Alzheimer dementia, bipolar disorder and schizophrenia.
St. John’s wort has been reported to cause worsening of symptoms in people with bipolar disorder or schizophrenia.
Taking St. John’s wort before driving or operating heavy machinery may pose a risk for people who experience sedation as a side effect.
St. John’s wort might increase the risk of cataracts.
The above does not include all the possible medical conditions or situations in which it may be unsafe to take St. John’s wort. Always consult a medical professional for medical advice.
National Center for Complementary and Integrative Health 17provides a summary of examples of medications, that when taken in combination with St. John’s wort, can potentially dangerously decrease the potency of the medications:
- Antidepressants
- Some heart medications, including digoxin and ivabradine
- Some HIV drugs, including indinavir and nevirapine
- Some cancer medications, including irinotecan and imatinib
- Warfarin, an anticoagulant (blood thinner)
- Certain statins, including simvastatin and the supplement red yeast rice
- Birth control pills
- Cyclosporine, which prevents the body from rejecting transplanted organs
And numerous others (some examples are listed below)
St. John’s Wort Interacts with Drugs & Supplements
Examples of drug interactions in which St. John’s wort may decrease the levels or effectiveness of the drug have been reported by experts. 18 These include:
- ANTACIDS – omeprazole (Prilosec)
- ADHD MEDICATIONS – methylphenidate (Concerta)
- ANTI-ANXIETY DRUGS – alprazolam (Xanax),
- ANTIDEPRESSANTS – norepinephrine/dopamine-reuptake inhibitor (NDRI) antidepressant and smoking cessation drug, bupropion (Wellbutrin)
- ANTIARRHYTHMICS, HEART FAILURE & ABNORMAL HEART RHYTHM MEDICATIONS – digoxin (Lanoxin), ivabradine (Corlanor), procainamide
- ANTI-DIABETICS – gliclazide (Diamicron, others)
- ANTIFUNGALS – voriconazole (Vfend)
- ANTI-SEIZURE MEDICATIONS used to treat epilepsy – phenytoin (Dilantin), mephenytoin (Mesantoin), phenobarbital (Luminal)
- ANTIVIRALS – boceprevir (Victrelis)
- PROTEASE INHIBITORS – indinavir (Crixivan), and non-nucleoside reverse transcriptase inhibitors (NNRTIs)
- BLOOD PRESSURE MEDICATIONS – reserpine (Raudixin)
- BLOOD THINNERS – warfarin (Coumadin), phenprocoumon (Marcoumar), rivaroxaban (Xarelto)
- BRONCHODILATORS – theophylline
- CHEMOTHERAPY DRUGS – docetaxel (Taxotere), imatinib (Gleevec), irinotecan (Camptosar)
- CONTRACEPTIVE DRUGS – combinations with St. John’s wort could result in unplanned pregnancy
- IMMUNOSUPPRESSANTS drugs that prevent organ rejection after transplantation such as a liver transplant, cyclosporine (Neoral, Sandimmune), tacrolimus (Prograf)
- MEDICATION FOR CERTAIN MOOD DISORDERS and schizophrenia, schizoaffective disorders – clozapine (Clozaril)
- PAIN MEDICATIONS & ANESTHETICS – fentanyl, methadone (Dolophine), oxycodone (Oxycontin), tramadol (Ultram), ketamine (Ketalar)
- PROSTATE MEDICATION that treat benign prostatic hyperplasia (BPH) and medications to prevent male-pattern baldness – finasteride (Proscar, Propecia)
- SLEEPING PILLS – zolpidem (Ambien)
- Certain STATINS that treat high cholesterol – atorvastatin, lovastatin, rosuvastatin and others
- VASODILATORS that treat pulmonary arterial hypertension – ambrisentan (Letairis)
Example of supplement combinations where St. John’s wort may decrease the levels or effectiveness of the supplement include:
- Digitalis and other herbs with similar cardiac effects
- Iron supplements – theoretically St. John’s wort could reduce absorption
- Red yeast rice supplements – theoretically St. John’s wort could reduce blood levels
Examples of drug interactions in which St. John’s wort may increase the levels or the potency of the drug include:
- ANTIHISTAMINES – fexofenadine (Allegra), might cause toxicity
- PLATELET INHIBITORS (anti-clotting agents) – clopidogrel (Plavix)
- PHOTOSENSITIZING AGENTS – aminolevulinic acid combinations with St. John’s wort might have additive effects
Taking St. John’s Wort with Certain Medications Can
Increase serotonin Levels in Potentially Dangerous Ways
If serotonin levels get too high, it can cause symptoms that range from mild shivering and diarrhea to full-blown serotonin syndrome, a potentially deadly condition with serious symptoms like severe muscle rigidity, fever, and seizures.
Some of the symptoms of serotonin syndrome include anxiety, agitation, changes in balance, confusion, hallucinations, fever, flushing, sweating, muscle twitching or stiffness, shivering or shaking, severe headache, upset stomach, vomiting, severe diarrhea, seizures, fast or abnormal heartbeat, as well as other symptoms including, in rare cases, death.
Examples of drug combinations with St. John’s wort that might elevate serotonin levels to potentially dangerous levels:
- Anti-migraine agents; triptans (sumatriptan)
- Antidepressants including SSRIs like Zoloft; serotonin norepinephrine reuptake inhibitors (SNRIs) like duloxetine (Cymbalta, Irenka) and buspirone (Buspar); tricyclic antidepressants like amitriptyline; and monoamine oxidase inhibitors (MAOIs) like selegiline, isocarboxazid, phenelzine, and tranylcypromine.
- Antipsychotics
- Anticonvulsants
- Antiparkinsonian agents
- Antibiotic linezolid (Zyvox)
- Cough and cold medications containing dextromethorphan
- Pain medications (meperidine, tramadol)
Examples of supplements that might boost serotonin to dangerous levels when combined with St. John’s wort include:
- 5-HTP (hydroxytryptophan)
- tryptophan
- black seed
- phenethylamine (PEA)
- SAMe
- acetyl-L-carnitine
The above lists do not provide all the possible medications for which St. John’s wort may interact. St. John’s wort may interact—in potentially dangerous ways—with medications not listed above. The above information cannot substitute for the medical advice of your physician. Anyone considering taking any St. John’s wort should consult with their physician beforehand.
Adverse Effects & Side Effects
It’s possible to take too much St. John’s wort leading to toxic effects. St. John’s wort may cause increased sensitivity to sunlight, especially when taken in large doses. 19 People taking high doses may need to wear protective clothing to prevent skin reactions including burning, itching rashes on sunlight exposed areas of the skin.
Rare Adverse Effects
Rare case reports of suicidal thinking and psychosis (loss of touch with reality) after taking St. John’s wort have appeared in the medical literature. But the FDA has not issued any warnings St. John’s wort supplements regarding suicide risks. Whereas the risk of suicidal thinking and behavior are prominent warnings given by the FDA regarding numerous pharmaceutical antidepressants.
The incidence of suicidal thinking and behavior linked to pharmaceutical medications resulted in the FDA putting out a black box warning regarding suicidality in children and adolescents being treated with certain antidepressant medications.
The FDA analyzed 372 randomized clinical trials of antidepressants pharmaceuticals involving nearly 100,000 participants. The “rate of suicidal thinking or suicidal behavior was 4% among patients assigned to receive an antidepressant, as compared with 2% among those assigned to receive placebo,” according to article published in the New England Journal of Medicine. Placebos are fake pills that contain the medication.
Other rare St. John’s wort side effects include hypomania such as could result in increased energy and activity, insomnia and lapses in judgement.
There are rare reports of palpitations, irregular heartbeat, changes in blood pressure, swelling, stomach upset, loss of appetite, diarrhea, nausea, vomiting, constipation, muscle or joint stiffness, tremor, muscle spasms and pain.
Sexual dysfunction occurs less frequently with St. John’s wort than with SSRI pharmaceutical antidepressants.
Cases of restlessness, insomnia, panic, and anxiety have been noted for some patients.
There have been isolated cases of a syndrome consisting of extreme anxiety, confusion, nausea, hypertension, and tachycardia within 2-3 weeks of taking St. John’s wort. 20 Authors of a 2001 article in the Canadian Journal of Psychiatry labeled two such cases as serotonin syndrome. 21
Severe serotonin syndrome is rare, according to a 2016 scientific paper. These physician researchers explain that this potentially dangerous condition is almost exclusively caused by mixing an MAOI antidepressant medication (see above) with an SSRI antidepressant medication (see above). 22
Withdrawal Symptoms
St. John’s wort has been associated with withdrawal effects similar to those found with its pharmaceutical counterparts—SSRIs and SNRIs antidepressant medications. 23
In people who take SSRIs and SNRIs for six weeks or longer, it was thought that abrupt discontinuation could lead to short-lived withdrawal symptoms—the so-called “antidepressant discontinuation syndrome.” But now it’s believed that the symptoms are not necessarily short-lived and far from mild. SSRI and SNRI withdrawal symptoms include insomnia, nausea, imbalance, sensory disturbances, elevation, arousal and more. This withdrawal syndrome reportedly happens in about 20 percent of people. 24, 25
A 2020 article published in American Psychological Association said: “The thinking in the medical community was that patients could wean off these drugs with minor side effects, but anecdotally, many patients have reported troubling mental and physical withdrawal symptoms that last for months or even years.” 26
The occurrence of withdrawal symptoms following discontinuation of St. John’s wort may not be related to dose or duration of use and are most likely to hit within two days after discontinuation but can occur one week or more after stopping the herb, according to experts at NatMed Pro. 27 These experts note that the St. John’s wort discontinuation symptoms that have been reported include headache, nausea, anorexia, dry mouth, thirst, cold chills, weight loss, dizziness, insomnia, paresthesia, confusion, and fatigue. 28, 29
Common Side Effects
“St. John’s wort is generally well tolerated,” when taken by mouth, according to expert peer-reviewed report. 30
Its most common side effects are diarrhea, dizziness, dry mouth, fatigue, headache, insomnia, restlessness, sleepiness, skin reactions and mild gastrointestinal discomfort and symptoms. 31, 32
Sexual dysfunction with St. John’s wort is less frequently reported than with SSRIs. 33
Less Common Side Effects
Lethargy, anxiety and agitation. 34
The above is not a complete list of possible St. John’s wort side-effects.
Possible Problems with Purity & Potency
All supplement can contain harmful impurities or lack potency. Safety-conscious supplement manufacturers use proven methods to batch test their final products for purity and potency. Some companies use third-party testing of the final product and offer a certificate of analysis demonstrating their products purity and potency to consumers who request such information.
To find out how to get the benefits of how St. John’s wort without side effects or drug interactions, click link below:
The information provided on this site is for educational purposes only; it is not medical advice and should not be substituted for professional medical advice. Always consult a qualified healthcare professional before starting or stopping any medications or supplements.
Care informed by the understanding that emotional and physical wellbeing are deeply connected
________________________________________________________________________________________________________________
By using MoodChangeMedicine.com, you agree to accept this website’s terms of use, which can be viewed here.
Citations
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- “St. John’s Wort”. National Center for Complementary and Integrative Health, National Institutes of Health. Last Updated: October 2020, accessed June 2024. ↩︎
- Qaseem A, Barry MJ, Kansagara D, et al. “Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians.” Ann Intern Med. 2016;164(5):350-9. View abstract. ↩︎
- Lane-Brown MM. “Photosensitivity associated with herbal preparations of St John’s wort (Hypericum perforatum).” Med J Aust. 2000 Mar 20;172(6):302. PMID: 10860103. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- Qaseem A, Barry MJ, Kansagara D, et al. “Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians.” Ann Intern Med. 2016;164(5):350-9. View abstract. ↩︎
- Hypericum Depression Trial Study Group. “Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial.” JAMA. 2002;287:1807-14. [PMID: 11939866] Crossref PubMed Google Scholar ↩︎
- Dean AJ, Moses GM, Vernon JM. “Suspected withdrawal syndrome after cessation of St. John’s wort.” Ann Pharmacother. 2003;37:150. View abstract. ↩︎
- Beckman SE, Sommi RW, Switzer J. “Consumer use of St. John’s wort: A survey of effectiveness, safety, and tolerability.” Pharmacotherapy. 2000;20:568-74. View abstract. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- “St. John’s Wort”. National Center for Complementary and Integrative Health, National Institutes of Health. Last Updated: October 2020, accessed June 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- Borrelli F, Izzo AA. 2009. “Herb-drug interactions with St John’s wort (Hypericum perforatum): an update on clinical observations.” Aaps J. 11(4):710–727. View PubMed Web of Science ®Google Scholar ↩︎
- Qaseem A, Barry MJ, Kansagara D, et al. “Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians.” Ann Intern Med. 2016;164(5):350-9. View abstract. ↩︎
- “St. John’s Wort”. National Center for Complementary and Integrative Health, National Institutes of Health. Last Updated: October 2020, accessed June 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- Lane-Brown MM. “Photosensitivity associated with herbal preparations of St John’s wort (Hypericum perforatum).” Med J Aust. 2000 Mar 20;172(6):302. PMID: 10860103. ↩︎
- Beckman SE, Sommi RW, Switzer J. “Consumer use of St. John’s wort: A survey of effectiveness, safety, and tolerability.” Pharmacotherapy. 2000;20:568-74. View abstract. ↩︎
- Parker V, Wong AH, Boon HS, Seeman MV. “Adverse reactions to St John’s Wort.” Can J Psychiatry. 2001. Feb;46(1):77-9. doi: 10.1177/070674370104600112. PMID: 11221494. ↩︎
- Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. “Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale.” Trends Pharmacol Sci. 2016 Nov;37(11):933-944. doi: 10.1016/j.tips.2016.09.001. Epub 2016 Sep 28. PMID: 27692695; PMCID: PMC5728156 ↩︎
- Dean AJ, Moses GM, Vernon JM. Suspected withdrawal syndrome after cessation of St. John’s wort. Ann Pharmacother 2003;37:150. View abstract. ↩︎
- Thompson C. “Discontinuation of antidepressant therapy: emerging complications and their relevance.” J Clin Psychiatry. 1998;59:541-8. ↩︎
- Agelink MW, Zitzelsberger A, Klisser E. “Withdrawal syndrome after discontinuation of venlafaxine.” Am J Psychiatry. 1997;154:1473-4. ↩︎
- Weir, Kirsten. “How hard is it to stop antidepressants? New research suggests antidepressant withdrawal symptoms might be more common, more severe and longer lasting than previously realized.” Monitor on Psychology. 2020. Vol. 51, No. 3. April 2020. American Psychological Association. Accessed February. 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- Dean AJ, Moses GM, Vernon JM. “Suspected withdrawal syndrome after cessation of St. John’s wort.” Ann Pharmacother. 2003;37:150. View abstract. ↩︎
- Beckman SE, Sommi RW, Switzer J. “Consumer use of St. John’s wort: A survey of effectiveness, safety, and tolerability.” Pharmacotherapy. 2000;20:568-74. View abstract. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- “St. John’s Wort Monograph” NatMed Pro Therapeutic Research Center database. current through 3/8/2024. Last modified on 3/7/2024. Accessed June, 2024. ↩︎
- Qaseem A, Barry MJ, Kansagara D, et al. “Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians.” Ann Intern Med. 2016;164(5):350-9. View abstract. ↩︎
- Hypericum Depression Trial Study Group. “Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial.” JAMA. 2002;287:1807-14. [PMID: 11939866] Crossref PubMed Google Scholar ↩︎
- Qaseem A, Barry MJ, Kansagara D, et al. “Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians.” Ann Intern Med. 2016;164(5):350-9. View abstract. ↩︎


Discussion
No comments yet.